- Overview
- Symptoms
- Causes & Risks
- Types of Eczema
- Locations on the Body
- Tests & Diagnosis
- Treatment
- Complications
- Living With
- View Full Guide
Advances in Eczema Treatment


Dupilumab for Kids
In June 2022, the FDA approved dupilumab (Dupixent) for children as young as 6 months. This biologic is an interleukin inhibitor and blocks the effects of proteins called cytokines that increase inflammation, weaken the skin barrier, and cause severe itching.
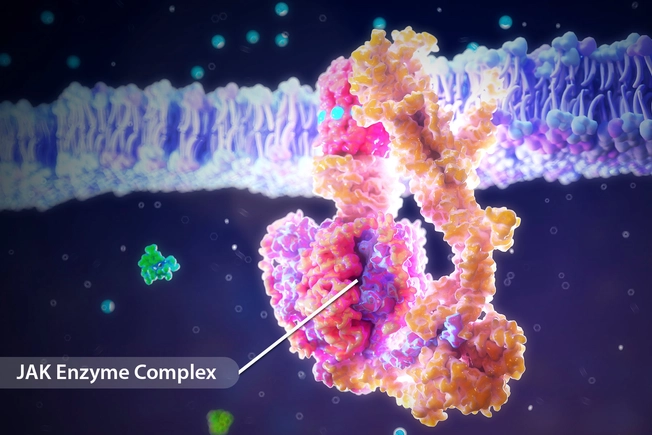
JAK Inhibitors
In the last year, the FDA has approved three new eczema treatments in a class of drugs called Janus kinase (JAK) inhibitors. A fourth drug is under review. These drugs block JAK enzymes, which play a part in driving the abnormal immune responses that lead to eczema symptoms.

Topical JAK Inhibitor
Ruxolitinib (Opzelura) is a medicated cream you spread on your skin. This is a good option if you need treatment other than a topical steroid, but your eczema isn’t bad enough for oral medication.

JAK Inhibitor Pills
Doctors can prescribe one of the two newly approved oral JAK medications, abrocitinib (Cibinqo) and upadacitinib (Rinvoq), if you have severe eczema on a large part of your body and you’ve already tried a systemic eczema medication like a biologic. You take them once a day.

Fourth JAK Inhibitor in Trials
If you can’t use cyclosporine as a treatment, there may be an alternative coming through the pipeline. Baricitinib (Olumiant) is another pill version of a JAK inhibitor still awaiting FDA approval. Doctors hope it can treat very severe eczema cases.
A New Biologic
Tralokinumab (Adbry) is a second biologic drug to treat moderate to severe eczema in people ages 12 and older. Tralokinumab is an injectable drug that blocks certain proteins responsible for the inflammatory response that causes eczema symptoms.
Stronger PDE4 Inhibitor
Roflumilast is a medicated cream that is a PDE4 inhibitor like crisaborole (Eucrisa), but studies show it may be even more potent. It is used to treat plaque psoriasis and atopic dermatitis in children and adults.

Other Topical Options
Tapinarof is the first in a new class of topical drugs called therapeutic aryl hydrocarbon receptor modulating agents (TAMA). These drugs inhibit two pro-inflammatory pathways that lead to itchy, inflamed skin. Tapinarof is approved for treating plaque psoriasis and is in phase III trials for atopic dermatitis.
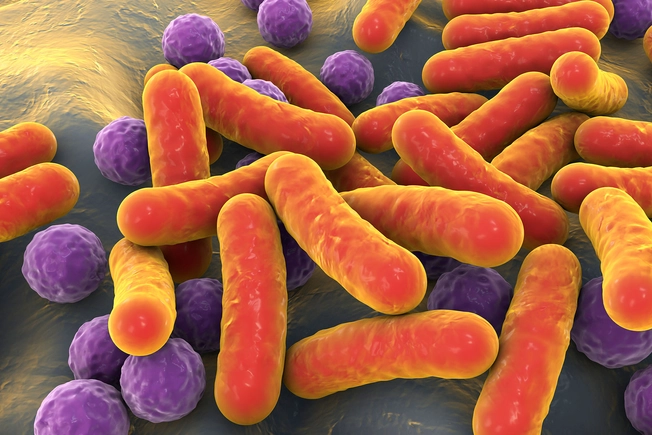
Bacteria Therapy
Researchers are looking into whether treating “bad” bacteria on the skin by using “good” bacteria will help prevent damage to the skin barrier. Some clinical trials show promise for bacteriotherapy creams or sprays that reduce symptoms with fewer side effects such as stinging.

Advances in Light Therapy
Light therapy, or phototherapy, uses a machine with UV rays to slow down the inflammatory response in skin. Treatment typically involves UVB light or a combination of UVB and UVA light. Researchers are doing clinical trials to better understand which UV light or lights will work best.
IMAGES PROVIDED BY:
- gpointstudio / Getty Images
- NANOCLUSTERING / SCIENCE PHOTO LIBRARY/ Getty Images
- danielle71 / Thinkstock / Getty Images
- Moussa81 / Getty Images
- LL28 / Getty Images
- Fly View Productions / Getty Images
- webphotographeer / Getty Images
- Tanja Ivanova / Getty Images
- Dr_Microbe / Getty Images
- Voisin / Phanie / Science Source
SOURCES:
Medscape: “FDA Approves Dupilumab for Children With Eczema Aged 6 Months to 5 Years,” “Baricitinib Reduces Signs and Symptoms of Cyclosporine-Resistant AD Up to 1 Year,” “Topical Roflumilast Approved for Psoriasis in Adults and Adolescents.”
American College of Clinical Pharmacology: “FDA Approves DUPIXENT (Dupilumab) for the Treatment of Adult Patients with Moderate-To-Severe Atopic Dermatitis Whose Disease is Not Adequately Controlled with Topical Prescription Therapies or when Those Therapies are Not Advisable.”
National Eczema Society: “Dupilumab.”
National Eczema Association: “JAK Inhibitors Are Coming and They Are the Biggest Eczema Development in Years,” “FAQ – Opzelura (Ruxolitinib) Cream,” “FAQ – Rinvoq (Upadacitinib),” “FAQ – Cibinqo (Abrocitinib),” “FAQ – Adbry (Tralokinumab-ldrm).”
American Journal of Managed Care: “Ruxolitinib Cream Approved for Short-term Treatment of Atopic Dermatitis.”
ClinicalTrials.gov: “Trial of PDE4 Inhibition With Roflumilast for the Management of Atopic Dermatitis (INTEGUMENT-I),” “Tapinarof for the Treatment of Atopic Dermatitis in Children and Adults (DMVT-505-3102),” “Broadband vs Narrowband Phototherapy for Eczema Trial Nested in the CACTI Cohort (BRONTE).”
Journal of the American Academy: “Tapinarof in the treatment of psoriasis: A review of the unique mechanism of action of a novel therapeutic aryl hydrocarbon receptor-modulating agent.”
JAMA Dermatology: “Use of Autologous Bacteriotherapy to Treat Staphylococcus aureus in Patients With Atopic Dermatitis.”
InformedHealth.org: “Eczema: Light therapy and oral medications.”